pFI: Single reagent assay by SFC, Glucose
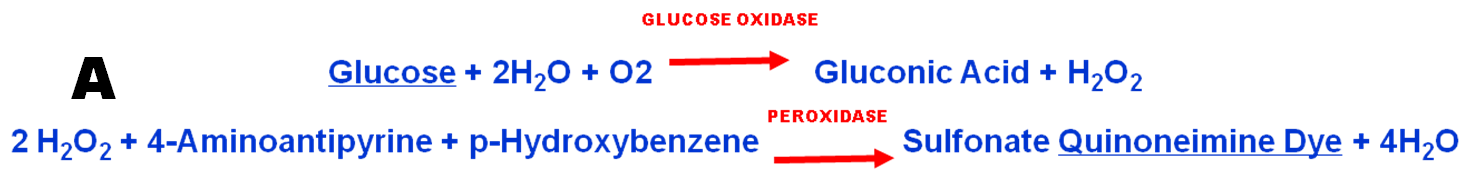
Enzymatic assay of glucose (A) is based on a two step reaction, producing red quinoeminine dye monitored at 500nm. The reactants are available in a single solution reagent kit (B).
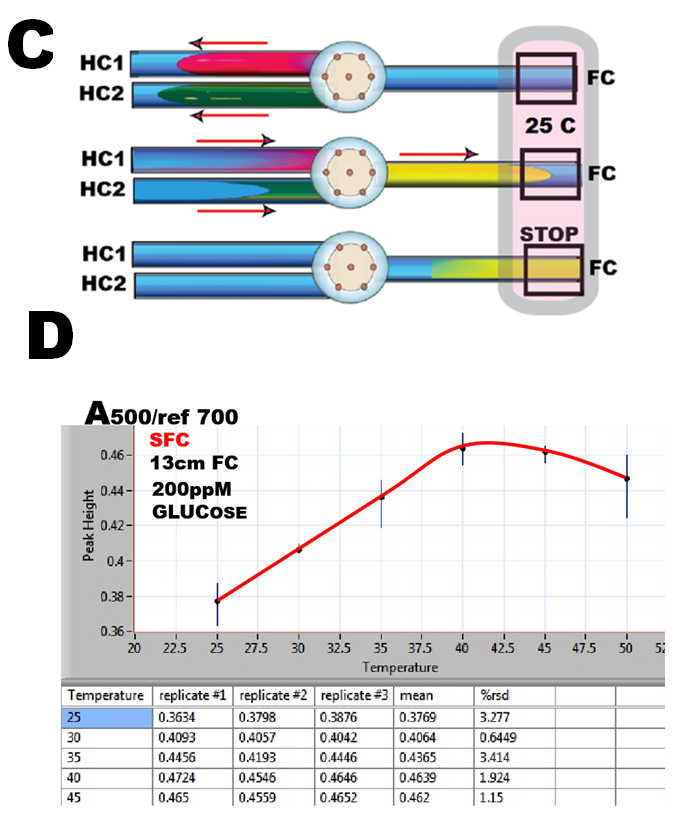
The pFI sequence of this assay (C) comprises three steps:
1) sample aspiration in HC1,
2) reagent aspiration in HC2,
3) simultaneous transport of reactants trough the confluence into the into flow cell, where a selected section of reaction mixture is arrested by stopping the flow for monitoring. This sequence is identical with the one used for assay of nitrite and therefore the assay protocol for nitrite (1.2.32.) picture (B) was used for all experiments described here.
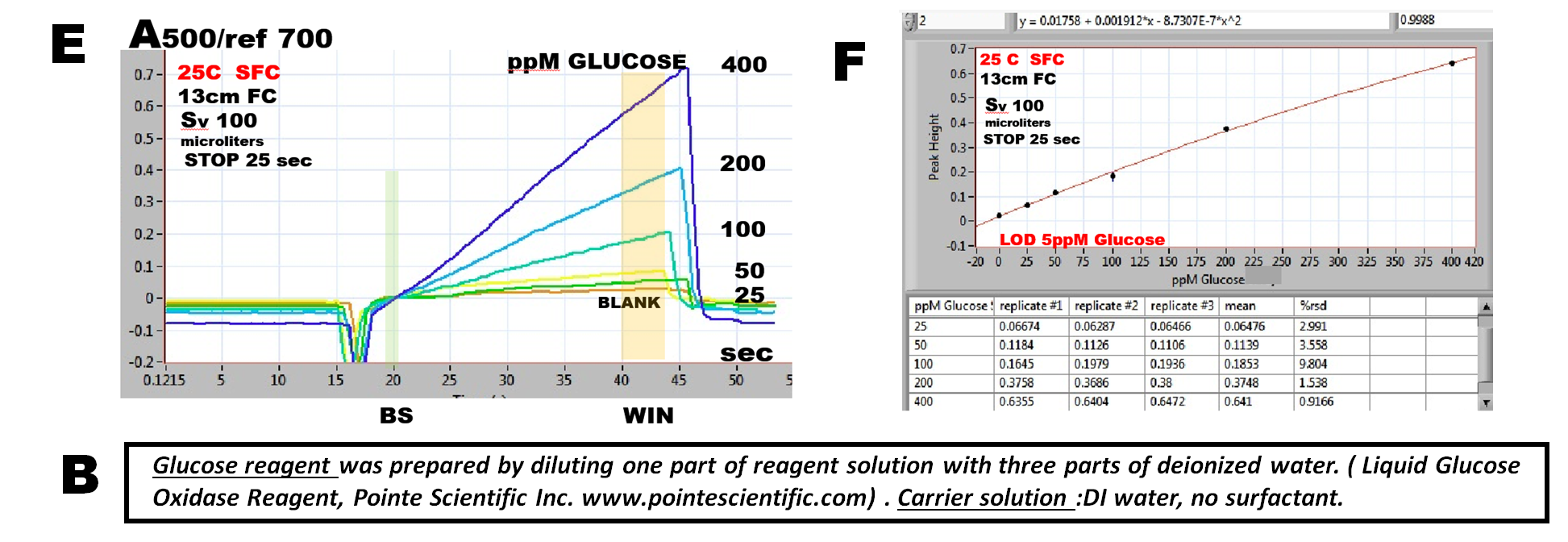
The influence of temperature was investigated first by using 100mcrL of 200ppM glucose standard and by measuring peak height while changing temperature of the flowcell between 25 to 50 C and by using incubation period of 25 seconds (D). Since little is gained in terms of peak height and sensitivity of assay be elevating temperature, 25 C was used for calibration experiments in order to ensure adequate thermostating of flow cell ( above ambient 21 C).
Reaction rate curves (E) recorded during 25sec long stop flow period show increasing slope, with the concentration of glucose, while in absence of glucose (BLANK) there is no increase of absorbance. The calibration graph (F) based on slope of reaction rate curve was obtained by subtracting A-value at SB time, from A-value at WIN time. Reproducibility of triplicate measurement (F), (average of 3.8% r.s.d.) is comparable with the traditional cFI or miniSI method.
Note the difference between reaction rates of nitrite (1.2.32.) of and glucose method. Since Gries reaction is very fast, the majority of the reaction product is formed on the way to detector, and therefore end point method had to be used for calibration, while calibration graph for glucose assay is based on evaluation of the linear portion of the reaction rate response.
1.2.33.